Sodium Cyanoborohydride
Other Names:
Sodium cyanotrihydridoborate
General Information:
Structure:
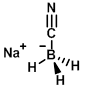
CAS Number: 25895-60-7
Molecular Weight: 62.84 g/mol
Appearance: White solid
Sodium cyanoborohydride (NaBH3CN) is a mild reducing agent that is commonly used in reductive aminations. The presence of the electron-withdrawing cyano (CN) group makes it less reactive than sodium borohydride (NaBH4). This reduced reactivity allows NaBH3CN to be employed at neutral or slightly acidic conditions for the selective reduction of iminium ions in the presence of ketones and aldehydes.
Reductive amination by NaBH3CN is sometimes done in the presence of certain Lewis acids (ex. Ti(OiPr)4, TiCl4, or ZnCl2). The aldehyde or ketone is typically prestirred with the amine in the presence of the Lewis acid, then treated with NaBH3CN. This procedure is useful in cases where imine formation is poor, when the use of excess amine isn't possible, or acidic conditions aren't well tolerated.
NaBH3CN can effectively be used in H2O and protic solvents (ex. MeOH or EtOH), unlike sodium triacetoxyborohydride (STAB) which is quickly degraded by H2O and protic solvents.
Common Uses:
Reagent for reductive amination reactions
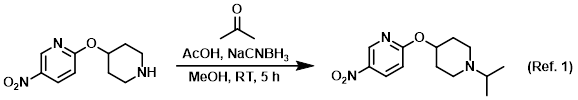
Procedure excerpt:
. . . the SM (1 equiv) and dry acetone (5 equiv). The solution was stirred at RT 1 h, after which time it was cooled to 0 C and treated with NaCNBH3 (1.5 equiv). The reaction was stirred . . .
Safety:
Sodium cyanoborohydride is a potential source of cyanide. Care must be taken when handling the reagent and disposing of the resulting waste.
References:
1) Patent Reference: WO2007084786, page 112, ![]() (9.4 MB)
(9.4 MB)
2) Wikipedia: Sodium cyanoborohydride (link)
3) www.sigmaaldrich.com: Sodium cyanoborohydride (link)
4) Burke, S. D.; Danheiser, R. L.; Handbook of Reagents for Organic Synthesis; Oxidizing and Reducing Reagents
5) Carey, F. A.; Sundberg, R. J.; Advanced Organic Chemistry, Part B: Reactions and Synthesis, 5th Edition